A new patent has been granted for an innovative sclerosing/regenerative solution created by Dr. Capurro in collaboration with the nuclear physicist, Engineer Di Salvo. The 20 claims made have all been deemed justified!
The Research Report issued by the UIBM is fully positive, in that the European examiner did not identify any previously patented similar product and judged the new solution to be novel, the result of sufficient inventive activity and endowed with the features necessary for industrial application with regard to all of the claims made.
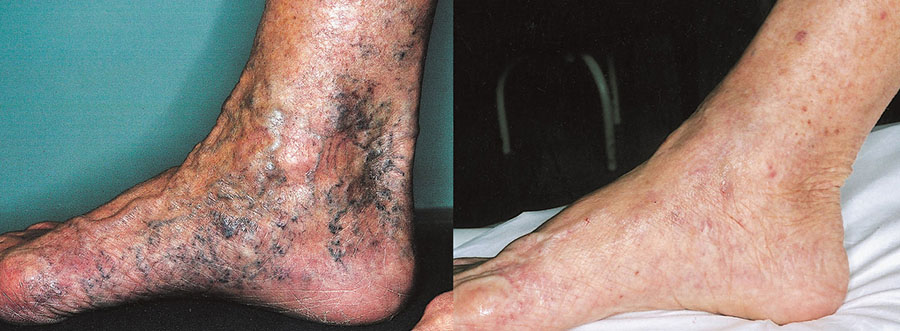
In Dr. Capurro's experience, this solution ensures the greatest safety and yields the best results in phlebotherapy. It should be remembered that the aim of TRAP is to achieve the complete disappearance of all the visible vessels and to restore correct venous hemodynamics in the lower limbs in all cases treated.
The regenerative solution described in the patent is the ideal choice for the treatment of the dilated venous circulation.
As is known, Dr. Capurro has revolutionized phlebology through his invention of TRAP (Thee-dimensional Regenerative Ambulatory Phlebotherapy), a technique which treats not only the effect but also the cause of varicose veins and telangiectasias by shrinking the caliber and strengthening the walls of the visible and non-visible vessels. In short, this method treats hemodynamic hypertension in the venous circulation of the lower limbs. In Dr. Capurro's view, varicose veins should not be obliterated by means of sclerotherapy, stripped out by means of phlebectomy, burnt with a high-frequency current or with a laser, or ligated. Indeed, these traditional two-dimensional approaches act only on the effect of the pathology and not on its anatomical cause, which lies in the perforating veins. The irrational destruction of the visible veins of the superficial circulation, as in the case of traditional techniques, eliminates the escape valve for the hemodynamic hypertension that these techniques are unable to treat.
The new patent is available to foreign laboratories and pharmaceutical companies.